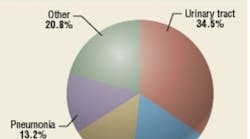
Hospital-acquired, or nosocomial, infections (Figure 1) have proven to be a persistent and sometimes tragic problem. If transmission by direct contact predominates, as many experts suggest, then surface-disinfection technologies should have a major impact in reducing-infection rates. But with more than a third of all nosocomial infections possibly involving airborne transmission at some point, the combination of surface and air disinfection should produce optimum results.
This article will examine the epidemiology and aerobiological pathways of airborne nosocomial infections and review air- and surface-disinfection technologies, including ultraviolet germicidal irradiation (UVGI). First, however, a brief synopsis of applicable guidelines, codes, and standards will provide background on current methods of nosocomial infection control.
GUIDELINES, CODES, AND STANDARDS
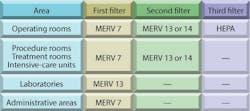
A variety of guidelines, codes, and standards offer details for designing health-care-facility ventilation systems.2,3,4,5 Some guidelines address specific problems, such as tuberculosis (TB), nosocomial infections, and surgical-site infections (SSIs).6,7,8,9 Supply-air filters typically are specified based on the American Society of Heating, Refrigerating and Air-Conditioning Engineers recommendations shown in Table 1. (MERV stands for "minimum-efficiency reporting value.")
Air recirculation is permitted in most hospital areas, including operating rooms (ORs) and intensive-care units (ICUs).3 General areas (GAs) often have recirculation rates typical of commercial office buildings, with about 15- to 25-percent outside air (OA). There are no specific requirements for filtration of recirculated air in GAs. Air-contaminant control often is accomplished with high rates of room air exchange using filtered 100 percent outside air. Typically, ORs have an air-change rate (ACH) of 12 to 25, with 12 ACH typically representing 100-percent OA and 25 ACH typically representing 5 ACH of OA and 20 ACH of recirculated air. Patient and intensive-care rooms typically have an ACH of 4 to 6, with 2 ACH of OA.4 The American Institute of Architects2 recommends 15 ACH for ORs, which appears to be the norm in the United States.
Figure 2 illustrates both the effectiveness of various rates of filtered outside air with complete air mixing and the effectiveness of recirculating the same airflow through a high-efficiency particulate-air (HEPA) filter. Note that the results are virtually identical, a fact that brings into question the universal reliance on 100-percent-OA systems, especially if, as discussed later, combined UVGI/filtration systems can approach HEPA-filter performance with lower energy costs.
Many mistakenly assume that systems designed and installed per code will produce sterile air. For analytical purposes, sterility often is assumed to mean six logs of reduction, which is the lower Y-axis limit in Figure 2. Technically, six logs of reduction implies sterility if and only if there are no survivors. Sterility of OR air may be difficult to achieve and impossible to prove.
HEPA filters typically are used in isolation rooms and other areas when air is recirculated.3 They also often are used to filter exhaust air from isolation rooms, laboratories, and other facilities. Codes and guidelines have specific requirements for the pressurization of ORs and isolation rooms that can be consulted for additional information. Finally, and perhaps most importantly, hospitals have detailed procedures for disinfection.5,10
All of the above approaches to infection control have proven to be reliable and effective in practice. However, based on ongoing research, certain improvements may be possible, particularly regarding nosocomial infections that are wholly or partly airborne.
AIRBORNE NOSOCOMIAL EPIDEMIOLOGY
Various sources estimate that between 2 million and 4 million nosocomial infections occur annually, resulting in 20,000 to 80,000 fatalities. The cost of nosocomial infections in the United States is estimated to be about $4 billion to $5 billion annually. Table 2 lists potentially airborne nosocomial agents and the number of annual cases, based on various sources. This list is by no means complete, as practically every pathogen and mold spore may become a nosocomial agent, and new pathogens often arise. Endogenous microbes are those that exist commensally in humans, but may be transmitted to susceptible individuals, usually the immuno-compromised. Filtration efficiency and UVGI dosages for 90-percent inactivation (D90) are summarized from "Aerobiological Engineering Handbook: A Guide to Airborne Disease Control Technologies."11
Airborne nosocomial infections are transmitted directly or indirectly through air and may cause respiratory (primarily pneumonia) and surgical-site infections. The degree to which the transmission of nosocomial infections is airborne is unknown. One source estimates that 10 percent of nosocomial infections are airborne, while another states that 16 percent of ICU infections result from airborne-pathogen transmission.12,13 These figures may be grossly underestimated, considering the etiology of nosocomial pneumonia and SSIs has not been studied or documented sufficiently. Some urinary-tract infections—and even some blood infections—may result from airborne microbes settling on equipment. In ICUs, almost a third of nosocomial infections are respiratory in nature, but not all are airborne; many are transmitted by contact.7,14 SSIs are non-respiratory, but may be partly airborne in origin—for example, when common microbes such as Staphylococcus and Streptococcus settle on open wounds, burns, or medical equipment.15
At a rate of about 0.1 to 0.3 per 1,000 discharges, SSIs are the most common type of nosocomial infection.8 The rates of nosocomial infections in a university hospital surgical ICU, surgical ward (SW), and medical ward (MW) were studied.16 The overall nosocomial infection rate was 14 percent, with infection rates of 42.5 percent for the surgical ICU, 19.6 percent for the SW, and 4.1 percent for the MW. Newer studies are available, but the results are similar.
Nearly 30 percent of sporadic cases of nosocomial pneumonia are caused by Legionella.17 Legionella is a common contaminant of water systems in hospitals; the formation of aerosols from contaminated water may be a major mode of spread. Legionella contamination is controlled most effectively with waterside disinfection and good plumbing practices.17,18
Hospital workers, as well as patients, are at risk of nosocomial infections, with worker fatalities reported. Health-care professionals routinely are exposed to contagious respiratory infections such as TB and influenza. In most cases in which medical workers have contracted respiratory infections from inhalation, the root cause has been inadequate local ventilation, malfunctioning systems and equipment, or administrative-control problems.19 TB infections among health-care workers are associated strongly with inadequate ventilation in general patient rooms and with the type and duration of work, but no associations have been identified with ventilation of isolation rooms.20 One of the greatest worker risks is infection with Bordetella pertussis, which causes both symptomatic and asymptomatic infections.21 Proper engineering design and maintenance and strict adherence to procedures can greatly reduce infection risk among health-care professionals. Routine vaccinations of health-care workers does not eliminate risk.
Newborns in neonatal intensive-care units (NICUs) are subject to aerobiological hazards similar in fashion to those of the immuno-compromised; however, the complicated etiologies cannot be reviewed in adequate detail here. (For additional information, see "Aerobiological Engineering Handbook: A Guide to Airborne Disease Control Technologies."11) The solutions for NICUs, however, essentially are the same as those for ICUs discussed later.
NOSOCOMIAL AEROBIOLOGY
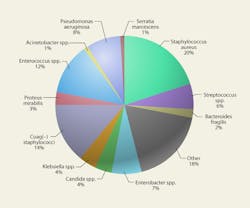
Any respiratory pathogen can produce a nosocomial infection. Most of the opportunistic pathogens that cause SSIs are at least partly airborne. Some environmental mold spores, such as Aspergillus, can cause opportunistic infections in the immuno-compromised. Figure 3 shows the variety of pathogens responsible for nosocomial infections. Nearly all are potentially airborne, although most infections probably are the result of direct contact and contact with equipment.
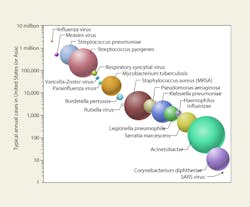
Figure 4 shows major airborne nosocomial bacteria and viruses ranked graphically by incidence and the relative proportion of their log-mean diameters. Ranking these pathogens in terms of fatalities—or costs—would be more difficult and might produce rather different hierarchies.
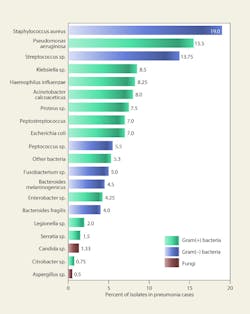
Figure 5 lists microbes isolated from the respiratory tracts of patients with nosocomial pneumonia in four separate studies.22 Viruses were not included in the studies; however, viral pneumonia primarily is attributable to influenza, respiratory syncytial virus, and parainfluenza. Between 13 and 54 percent of the pneumonia cases were polymicrobial in nature. Roughly 75 percent of the microbes were potentially airborne, although most of the infections probably resulted from contaminated ventilators, intubation, or direct contact.
Levels of airborne microbes are not routinely checked in hospitals; however, a variety of studies have indicated that the air in hospital areas rarely, if ever, is sterile. In a study of airborne microbial contamination in the OR and ICUs of a surgery clinic, bacterial concentrations of 150 to 250 cfu per cubic meter were measured.23 The most frequently isolated microorganisms included Staphylococcus epidermis, S. haemolyticus, Enterococcus spp., Enterobacter, Pseudomonas spp., Micrococcus, Corynebacteria, and Streptococcus faecalis. In a study of mold spores in the air of a hospital ward, 22 different types of spores, with total concentrations of 175 to 1,396 spores per cubic meter, were found.24 The most frequently isolated were Cladosporium, Ustilago, and various basidiospores. For AspergillusPenicillium spores, the concentration was higher indoors than outdoors, although for most spores, lower levels were found indoors, with a mean indoor/outdoor ratio of 1-to-4.
Patients may bring airborne infections into waiting areas. In one unusual case, three children who arrived at a pediatrician's office an hour after an infectious child left developed measles.
The microbes most frequently cultured in OR air include Staphylococcus epidermis and S. aureus. Streptococcus pyogenes has been found in about 15 percent of preoperative throat swabs from patients.25 The number of people in an operating suite influences counts of airborne bacteria.26 Conversation can increase the bacterial load of air and contaminate the facemasks of surgeons and nurses (9 to 10 percent of those measured postoperatively).27 A person may shed 3,000 to 50,000 microorganisms per minute, depending on activity and the effectiveness of protective clothing. Also, people shed skin squames, which may contain bacteria, continuously.
AEROBIOLOGICAL PATHWAYS OF NOSOCOMIAL INFECTIONS
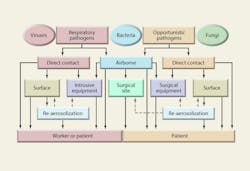
Major aerobiological pathways through which patients and health-care workers become infected with airborne nosocomial pathogens are shown in Figure 6. Only first- and second-order pathways are shown, although it is possible for a microbe to become reaerosolized several times or pass from person to person before causing an infection. Not every pathway is shown, and not every pathway shown is probable; however, Figure 6 illustrates the potential complexity of the aerobiological etiology of nosocomial infections.
Facemasks may not fully protect surgeons from aerosolized blood pathogens. Respirable (less than 5 µm) aerosols containing blood can be generated in an OR during surgery through the use of common surgical power tools.
Methicillin-resistant Staphylococcus aureus (MRSA) is a major nosocomial pathogen in many hospitals and is being isolated with increased frequency. According to one study, it is the most frequently isolated airborne microbe.13 The major sources of S. aureus in hospitals are septic lesions and carriage sites of patients and personnel. Anterior nares are the most common carriage site, followed by the perineal area. Although the principal mode of transmission is transiently contaminated hands of hospital personnel, airborne MRSA plays a role in respiratory-tract MRSA infections. MRSA has been found in air samples collected in single-patient rooms and has been isolated from sinks, floors, and bed sheets, as well as from patients' hands. MRSA recirculation in air is enhanced by activity in rooms, including the changing of bed sheets.
The virus that causes severe acute respiratory syndrome (SARS) is one of the most hazardous nosocomial agents to hospital personnel, although an outbreak has yet to occur in the United States. Evidence suggests aerosol transmission through ventilation systems, although the major transmission routes are close proximity airborne-droplet infection and close-contact infection. In previous outbreaks, index patients caused secondary infections in medical staff and inpatients. Hospital outbreaks of SARS typically occur within one week of the admission of a SARS patient and before isolation measures are implemented.28 Nosocomial transmission has been halted effectively through enforcement of standard procedures for controlling the transmission of high-risk airborne infections.
Nosocomial infections from fungal spores, such as Aspergillus, can occur when construction barriers are inadequate. Dust above suspended ceiling panels can be a major source of mold spores. Local air filtration is essential during renovation projects. The use of mobile HEPA-filter units is common and can reduce local airborne-spore levels significantly.
Figures 7 and 8 list levels of bioaerosols sampled in various health-care facilities. These levels generally are lower than they would be in typical office buildings, but higher than would be predicted for buildings with high-efficiency filters. For hospital air, the World Health Organization recommends relatively relaxed limits of 100 cfu per cubic meter for bacteria and 50 cfu per cubic meter for fungi; however, many facilities would fail to meet even these.29 Considering that environmental fungal spores should be completely removed per filtration guidelines, the presence of any fungal spores in an OR should warrant investigation. According to the criteria of Federal Standard 209E, Airborne Particulate Cleanliness Classes in Cleanrooms and Clean Zones, conventionally ventilated ORs rank less than Class 3.5.13 A limit of 10 cfu per cubic meter, based on the International Organization for Standardization Class 7 cleanroom limit (EU Grade B) used in the pharmaceutical industry and as a target for ultraclean ventilation systems, probably would be a more appropriate criterion for hospital ORs and ICUs. The industry needs more research correlating airborne bacterial concentrations in ORs with SSI rates (Figure 9).
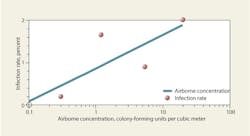
A study on Aspergillosis in hospitals18 reviews literature on infection rates and summarizes the levels shown in Figure 9, suggesting that a threshold may exist at about 0.1 cfu per cubic meter, below which infection rates may be negligible. Similar correlations between airborne bacteria and infection rates have been theorized and mentioned anecdotally in literature; however, limited quantitative data are available. One study30 suggests the respiratory-tract infection rate approaches 0.7 percent as airborne concentrations of bacteria decrease toward 0.028 cfu per cubic meter.
Bioaerosols released by people are thought to tend to float around them before precipitating downward.31Figure 10 illustrates potential sources. Flatulence may explain how bacteria can get from the colon of OR personnel into the open wound of a surgery patient. Facemasks are known to be poor filters, with one source suggesting doubling up facemasks, although this still is not as effective as an N95 facemask.32
The etiology of nosocomial SSIs is difficult to trace and often may include distinctive pathways and unusual microbes. The subject is far too complex to be addressed adequately in this article. For additional information, see the references listed at the end of this article.
CONTROL OPTIONS
Although current methods of controlling nosocomial infections, including isolation rooms and disinfection procedures, have proven successful, there certainly is room for improvement, especially in the United States. New nosocomial hazards demand renewed interest in both causes and solutions. Topics of discussion should include the establishment of specific airborne-contamination limits, the increased use of air sampling to identify nosocomial sources, the use of UVGI on a larger scale, the exploration of green technologies and more energy-efficient methods of controlling airborne microorganisms, and the use of new antimicrobial materials.
Laminar-airflow systems with 16 to 17 ACH supplied through HEPA filters are capable of holding OR airborne concentrations below 10 cfu per cubic meter.33 The use of HEPA filters, however, may represent overkill when applied for the control of airborne microbes. In one OR test, a HEPA filter provided no better reduction in airborne bacterial load than a 95-percent dust-spot filter.34 Furthermore, the combination of UVGI and high-efficiency filters in the MERV-13to-15 range may be able to provide performance virtually equivalent to HEPA filtration, thus offering health-care facilities the possibility of reducing energy costs without increasing health risks.
A major potential source of fungal contamination in hospitals is filter bypass and maintenance problems. Because they are used to filter large quantities of outside air, MERV 7 to MERV 13 filters are likely to accumulate spores. Filters should be checked for bypass, and maintenance procedures requiring shutdown of fans should be followed diligently; otherwise, spores may enter ventilation systems and accumulate in carpeting and furnishings. Periodic surface sampling of cooling coils and drain pans can help identify potential problems. Sampling carpets for contamination is another prudent practice, with heavily contaminated carpets removed, rather than cleaned.
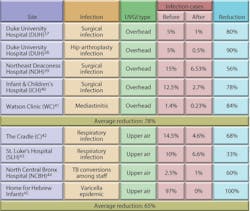
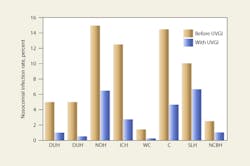
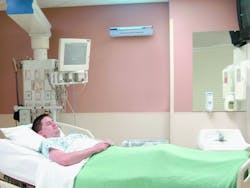
UVGI systems have been in use in ORs since at least 1937. Table 3 shows the results of some of these applications. Reductions in post-operative infection rates of 24 to 44 percent have been demonstrated.35 Overhead UVGI systems have been used to control SSIs, while upper-air UVGI systems have been used to control respiratory infections. The Home for Hebrew Infants in New York was able to bring a halt to a Varicella epidemic using UVGI. One study showed that UVGI can reduce airborne microbial concentrations to below 10 cfu per cubic meter in an OR.36 Despite these early successes, UVGI continues to be largely ignored as an option by the health-care field and regulatory agencies. Figure 11 illustrates the results of several studies from Table 3. Upper-air UVGI systems, such as the one shown in Photo A, are easy to install, but require experienced consultation. In-duct, forced-air UVGI systems may be the safest, most effective, and most efficient to install in large facilities, but because one has yet to be installed in a hospital, no data regarding reduction of nosocomial infection rates are available.
Intrusion of airborne fungi and environmental bacteria can cause contamination of carpets and furniture. Contamination may come from visitors and cause accumulation of microbes in areas such as ICUs. In one hospital studied by the author, heavy bacterial contamination was found in a lunchroom carpet, from which doctors and nurses apparently were carrying bacteria on the soles of their shoes into and around the ICU. The bacteria may have become re-aerosolized in the procedure rooms during activity. It was recommended that the carpet be removed from the lunchroom and surrounding areas.
A novel application of UVGI is the use of systems to decontaminate surfaces, including floors, carpets, and equipment, after hours. Such systems can be installed to irradiate an entire ICU or OR while it is unoccupied. Motion detectors automatically shut down the systems whenever someone enters the room.
Also emerging is the use of pulsed-light systems for surface disinfection.46 Pulsed light with the Ultraviolet C component removed has the unique ability to destroy bacterial cells on the surface of skin with minimal hazard to human cells. High-intensity pulsed light can cause sudden cell rupture from instantaneous heating, but skin cells, locked into a tight matrix, remain undamaged. Available for hand decontamination, this technology may be adaptable for overhead surgical-site disinfection.
Evidence suggests that air ionization can help reduce the bacterial load of air and control airborne nosocomial infections. Antimicrobials are a promising development for the health-care industry, with protective clothing, dressings, surfaces, and medical equipment increasingly being made available.
Ventilation-system performance may change over time, and reversals of airflow direction may occur between zones, resulting in contaminant intrusion in ICUs and ORs. Many hospitals have wards and interconnected facilities with separate ventilation systems designed to keep critical areas under slight positive pressure with respect to hallways and public-access areas. In one health-care facility, the author found air pressurization had been reversed because of creeping airflow imbalances and that contaminants were flowing from GAs into the ICU zone. It would be prudent for building engineers to check pressurization/airflow direction periodically, which can be done with smoke testing and by other means.
The change that perhaps would have the greatest impact on nosocomial infections in this country is the establishment of national standards concerning aerobiological quality in hospitals. Routine air sampling, such as that recommended by the National Institute of Health Sciences in Japan, would provide a clearer picture of the relationship between airborne microbes and infection rates. More research on the aerobiological pathways and etiology of nosocomial infections is needed so that appropriate solutions can be explored and implemented better.
REFERENCES
- CDC. (1996). National Nosocomial Infections Surveillance (NNIS) System report, data summary from October 1986 through April 1996, issued May 1996. American Journal of Infection Control, 24, 380-388.
- AIA. (2001). Guidelines for design and construction of hospital and health care facilities. Washington, DC: American Institute of Architects.
- ASHRAE. (2003). HVAC design manual for hospitals and clinics. Atlanta: American Society of Heating, Refrigerating and Air-Conditioning Engineers.
- ASHRAE. (2003). ASHRAE handbook—HVAC applications. Atlanta: American Society of Heating, Refrigerating and Air-Conditioning Engineers.
- CDC. (2003). Guidelines for environmental infection control in health-care facilities. Atlanta: U.S. Department of Health and Human Services Centers for Disease Control and Prevention.
- CDC. (2005). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. Atlanta: U.S. Department of Health and Human Services Centers for Disease Control and Prevention.
- Wenzel, R.P. (1981). CRC handbook of hospital acquired infections. Boca Raton, FL: CRC Press.
- Mangram, A.J., Horan, T.C., Pearson, M.L., Silver, L.C., Jarvis, W.R., & HICPAC. (1999). Guideline for prevention of surgical site infection. American Journal of Infection Control, 27, 98-134.
- Tablan, O.C., Anderson, L.J., Arden, N.H., Beiman, R.F., Butler, J.C., & HICPAC. (1994). Guideline for the prevention of nosocomial pneumonia. American Journal of Infection Control, 22, 247-292.
- Gruendemann, B.J., & Stonehocker, S. (2001). Infection prevention in surgical settings. Philadelphia: W.B. Saunders.
- Kowalski, W.J. (2006). Aerobiological engineering handbook: A guide to airborne disease control technologies. New York: McGraw-Hill.
- Eickhoff, T.C. (1994). Airborne nosocomial infection: A contemporary perspective. Infection Control and Hospital Epidemiology, 15, 663-672.
- Durmaz, G., et al. (2005). The relationship between airborne colonization and nosocomial infections in intensive care units. Journal of Chemotherapy, 39, 465-471.
- Wilson, J. (2001). Infection control in clinical practice. Edinburgh: Balliere Tindall.
- Fletcher, L.A., Noakes, C.J., Beggs, C.B., & Sleigh, P.A. (2004, May). The importance of bioaerosols in hospital infections and the potential for control using germicidal ultraviolet irradiation. Proceedings of the 1st seminar on Applied Aerobiology, Murcia, Spain.
- Munzinger, J., Buhler, M., Geroulanos, S., Luthy, R., & Graevenitz, A.V. (1983). Nosocomial infections in a university hospital. Schweiz Med Wochenschr, 113, 1782-1790.
- Hart, C.A., & Makin, T. (1991). Legionella in hospitals: A review. The Journal of Hospital Infection, 18 Suppl A, 481-489.
- Streifel, A.J. (1996). Controlling aspergillosis and Legionella in hospitals. Boca Raton, FL: CRC Press.
- Castle, M., & Ajemian, E. (1987). Hospital infection control: Principles and practice. New York: John Wiley & Sons.
- Menzies, D., Fanning, A., Yuan, L., & Fitzgerald, J.M. (2000). Hospital ventilation and risk for tuberculosis infection in Canadian health care workers. Annals of Internal Medicine, 133, 779-789.
- Wright, S.W., Decker, M.D., & Edwards, K.M. (1999). Incidence of pertussis infection in healthcare workers. Infection Control and Hospital Epidemiology, 20, 120-123.
- CDC. (1997). Guidelines for prevention of nosocomial pneumonia. Morbidity and Mortality Weekly Report, 46, 1-79.
- Holcatova, I., Bencko, V., & Binek, B. (1993). Indoor air microbial contamination in the operating theatre and intensive care units of the surgery clinic. Proceedings of Indoor Air ‘93, Helsinki, Finland, pp. 375-378.
- Tormo, M.R., Gonzalo, G.M.A., Munoz, R.A.F., & Silva, P.I. (2002). Pollen and spores in the air of a hospital out-patient ward. Allergologia et Immunopathologia, 30, 232-238.
- Dubuc, F., Guimont, A., Roy, L., & Ferland, J.J. (1973). A study of some factors which contribute to surgical wound contamination. Clinical Orthopaedics, 96, 176-178.
- Hambraeus, A., Bengtsson, S., & Laurell, G. (1977). Bacterial contamination in a modern operating suite. The Journal of Hygiene, 79, 121-132.
- Ritter, M.A. (1984). Conversation in the operating theatre as a cause of airborne bacterial contamination. The Journal of Bone & Joint Surgery, 66, 472.
- Ho, P.L., Tang, X.P., & Seto, W.H. (2003). SARS: Hospital infection control and admission strategies. Respirolog, 8, S41-S45.
- WHO. (1988). Indoor air quality: Biological contaminants. Copenhagen, Denmark: World Health Organization.
- McGuckin, M.B., & Kelsen, S.G. (1981). Surveillance in a surgical intensive care unit: Patient and environment. Infection Control, 2, 21-25.
- Sherertz, R.J., Bassetti, S., & Bassetti-Wyss, B. (2001). "Cloud" health care workers. Emerging Infectious Disease, 7, 241-244.
- Derrick, J.L., & Gomersall, C.D. (2005). Protecting healthcare staff from severe acute respiratory syndrome: Filtration capacity of multiple surgical masks. The Journal of Hospital Infection, 59, 365-368.
- Friberg, B., & Friberg, S. (2005). Aerobiology in the operating room and its implications for working standards. Proceedings of the Institution of Mechanical Engineers, Part H, Journal of Engineering in Medicine, 219, 153-160.
- Luciano, J.R. (1977). Air contamination control in hospitals. New York: Plenum Press.
- Goldner, J.L., & Allen, B.L. (1973). Ultraviolet light in orthopedic operating rooms at Duke University. Clinical Orthopaedics, 96, 195-205.
- Berg-Perier, M., Cederblad, A., & Persson, U. (1992). Ultraviolet radiation and ultra-clean air enclosures in operating rooms. The Journal of Arthroplasty, 7, 457-463.
- Kraissl, C.J., Cimiotti, J.G., & Meleney, F.L. (1940). Considerations in the use of ultra-violet radiation in operating rooms. Annals of Surgery, 111, 161-185.
- Lowell, J.D., Kundsin, R.B., Schwartz, C.M., & Pozin, D. (1980). Ultraviolet radiation and reduction of deep wound infection following hip and knee arthroplasty. In: Airborne Contagion. New York: New York Academy of Sciences.
- Overholt, R.H., & Betts, R.H. (1940). A comparative report on infection of thoracoplasty wounds. Journal of Thoracic Surgery, 9, 520-529.
- Del Mundo, F., & McKhann, C.F. (1941). Effect of ultra-violet irradiation of air on incidence of infections in an infant's hospital. American Journal of Diseases of Children, 61, 213-225.
- Brown, I.W., Moor, G.F., Hummel, B.W., Marshall, W.G., & Collins, J.P. (1996). Toward further reducing wound infections in cardiac operations. The Annals of Thoracic Surgery, 62, 1783-1789.
- Sauer, L.W., Minsk, L.D., & Rosenstern, I. (1942). Control of cross infections of respiratory tract in nursery for young infants. The Journal of the American Medical Association, 118, 1271-1274.
- Higgons, R.A., & Hyde, G.M. (1947). Effect of ultra-violet air sterilization upon incidence of respiratory infections in a children's institution. New York State Journal of Medicine, 47.
- EPRI. (1997). UVGI for TB infection control in a hospital. Palo Alto, CA: Electric Power Research Institute.
- Wells, W.F. (1955). Airborne contagion and air hygiene: An ecological study of droplet infections. Cambridge, MA: Harvard University Press.
- Wekhof, A., Trompeter, F-J, & Franken, O. (2001, June). Pulsed UV disintegration (PUVD): A new sterilisation mechanism for packaging and broad medical-hospital applications. Paper presented at The First International Conference on Ultraviolet Technologies, Washington, DC.
Wladyslaw J. Kowalski, PE, PhD, is vice president of Immune Building Systems Inc., a provider of advanced design and analysis for the protection of buildings against airborne-disease transmission, mold contamination, nosocomial infections, and the threat of biological-weapon agents. He conducts research for the Indoor Environment Center at The Pennsylvania State University and is chairman of the Air Treatment Group of the International Ultraviolet Association. He can be contacted at [email protected].