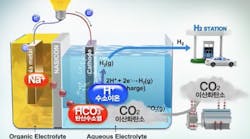
Last month, I wrote about a breakthrough in fuel cell technology achieved by researchers at South Korea’s Ulsan National Institute of Science and Technology (UNIST). They had developed an electrochemical cell that uses CO2 to produce electricity – thereby eliminating what is arguably the major contributor to climate change – with hydrogen as its byproduct.
Hydrogen is a zero-emission (i.e., clean) fuel when burned with oxygen and it has one of the highest energy density values per mass of any fuel (120 to 142 MJ/kg, compared to propane or gasoline at 46.4 MJ/kg). It is, however, generally expensive to produce and dangerous to transport. Of course, producing hydrogen from water by using, rather than generating, electricity is not a new technology.
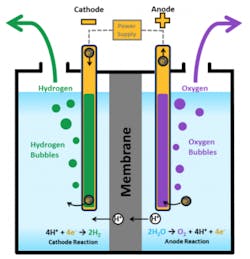
Electrolysis has been around since at least the early 19th century, when two Englishmen – a chemist and a surgeon – used the recently-invented voltaic pile (the first real battery) to separate hydrogen and oxygen from fresh water. The principal is fairly simple: Like a fuel cell, an electrolyzer consists of an anode and a cathode separated by an electrolyte. In this case, the electricity is applied to the two electrodes. The water reacts at the anode to form oxygen and positively charged hydrogen ions, which then move through the electrolyte to the cathode, where they combine with the electrons from the power source to form hydrogen.
What if we could use seawater?
Even today, producing hydrogen from electrolysis is a common commercial practice. The limitation, other than cost, is that the electrolytic process generally requires relatively pure water. Since clean water is already a scarcity in much of the world, widespread electrolysis using those water supplies would only exacerbate an already critical situation. That’s why I found the UNIST technology to be so exciting, since it has the potential to use seawater, rather than clean water, as a fuel to produce electricity and hydrogen, and also to reduce CO2.
Source: U.S. Dept. of Energy
Now, not to be undone, a team of scientists at Stanford University and Beijing University of Chemical Technology have developed an electrolyzer that produces hydrogen from seawater using solar power. In a new paper entitled “Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels,” and published in the Proceedings of the National Academy of Sciences of the United States of America, researchers led by Prof. Yun Kuang, Dr. Michael J. Kenney, and Prof. Hongjie Dai, explain how they are able to use corrosive seawater without having to first desalinate it, an extremely costly and energy intensive process.
Take it from me, a coastal resident of South Florida, salt water is hard on metal. We replace our outdoor furniture – both epoxy-coated steel and anodized aluminum frames – every few years. And it’s no different for the positively-charged metal anode in the electrolyzer. The Kuang-Kenney-Dai team’s solution was to develop a multi-layer electrode with high chloride corrosion resistance and a negatively-charged catalyst that would repel negatively-charged salt molecules. The Stanford electrolyzer achieved a current density of 400 mA/cm2 at 2.1 V in real seawater, with no obvious reduction in activity after 1,000 hours of testing.
Stated simply, the potential of this process is huge. Imagine commercial power plants in the future using only seawater and sunlight to generate abundant electricity! All pollution-free!
A regular contributor to HPAC Engineering and a member of its editorial advisory board, the author is a principal at Sustainable Performance Solutions LLC, a south Florida-based engineering firm focusing on energy and sustainability. For more, click here.